DRIVE-AHEAD resistance profile
DELSTRIGO® DRIVE-AHEAD resistance profile
Resistance profile of doravirine, 3TC and TDF in a 48-and 96-week study of patients with HIV-1:1,*
Observed occurrence of doravirine- or EFV- emergent associated resistance substitutions in the doravirine and the EFV/FTC/TDF treatment arms, respectively, in the resistance analysis subset at Week 48 (HIV-1 RNA >400 copies per mL at virologic failure or at early study discontinuation and having resistance data):1
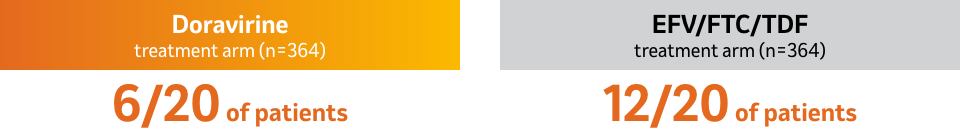
New cases of genotypic or phenotypic resistance to doravirine between Weeks 48 and 96:1
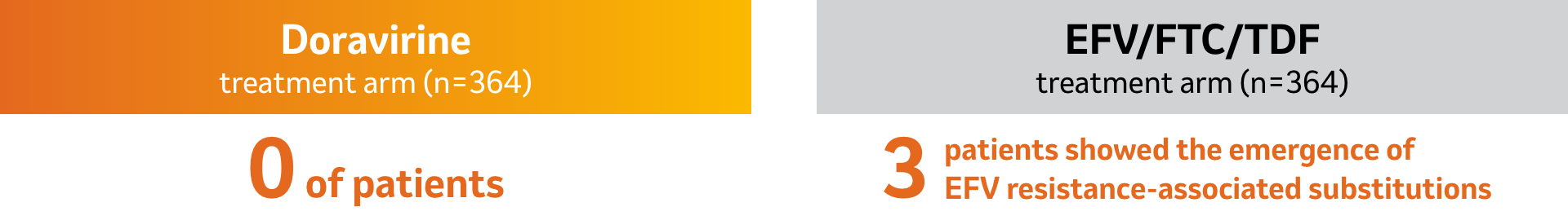
Emergent doravirine resistance-associated substitutions in RT included one or more of the following: A98G, V106A, V106I, V106M/T, Y188L, H221Y, P225H, F227C, F227C/R, and Y318Y/F.1
Resistance profile of doravirine, 3TC and TDF in a 48-and 96-week study of patients with HIV-1:1,*
- In a pooled analysis of antiretroviral-naïve subjects who received doravirine, 3TC, and TDF, genotyping was performed on plasma HIV-1 isolates from all patients with HIV-1 RNA >400 copies per mL at confirmed virologic failure or at time of early study drug discontinuation.
- Genotypic resistance developed in 8 evaluable subjects who received DELSTRIGO® through Week 96. 3TC and TDF resistance-associated substitutions were RT M41L (n =1), A62A/V (n=1), K65R (n=2), and M184V/I (n=4).
- Between Weeks 48 and 96, genotypic resistance to FTC or TDF developed in 1 patient who received EFV/FTC/TDF; the emergent resistance-associated substitution subject was V118I.
- In DRIVE-AHEAD, no additional genotypic or phenotypic resistance to doravirine, 3TC or TDF was identified in patients in the doravirine treatment arm between Weeks 48 and 96
* Clinical significance has not been established.
3TC=lamivudine; TDF=tenofovir disoproxil fumarate; EFV=efavirenz; FTC=emtricitabine; RT=reverse transcriptase; ART=antiretroviral therapy
Reference: 1. DELSTRIGO® Product Monograph. Merck Canada Inc. December 10, 2024.
CA-DOR-00155