DRIVE-AHEAD trial
DELSTRIGO® DRIVE-AHEAD trial*
DELSTRIGO® was studied in a Phase 3 non-inferiority trial in antiretroviral treatment-naïve patients1
Randomized, international, multicenter, double-blind, active-controlled trial to assess efficacy and safety profile (48- and 96-week duration) in antiretroviral treatment-naïve patients2

Entry criteria:1
- Adults ≥18 years of age
- Plasma HIV-1 RNA ≥1000 copies/mL (within 45 days before study treatment)
- Naïve to antiretroviral therapy
- No documented or known resistance to any of the study drugs
- Calculated creatinine clearance ≥50 mL/min
DELSTRIGO® demonstrated a non-inferior proportion of patients achieving HIV RNA <50 copies/mL at 48 weeks vs. EFV/FTC/TDF (primary endpoint) and similar efficacy at 96 weeks (using the FDA snapshot approach supporting the non-inferiority established at Week 48; secondary endpoint)2,*
Proportion of patients achieving HIV RNA <50 copies/mL at 48 and 96 weeks*
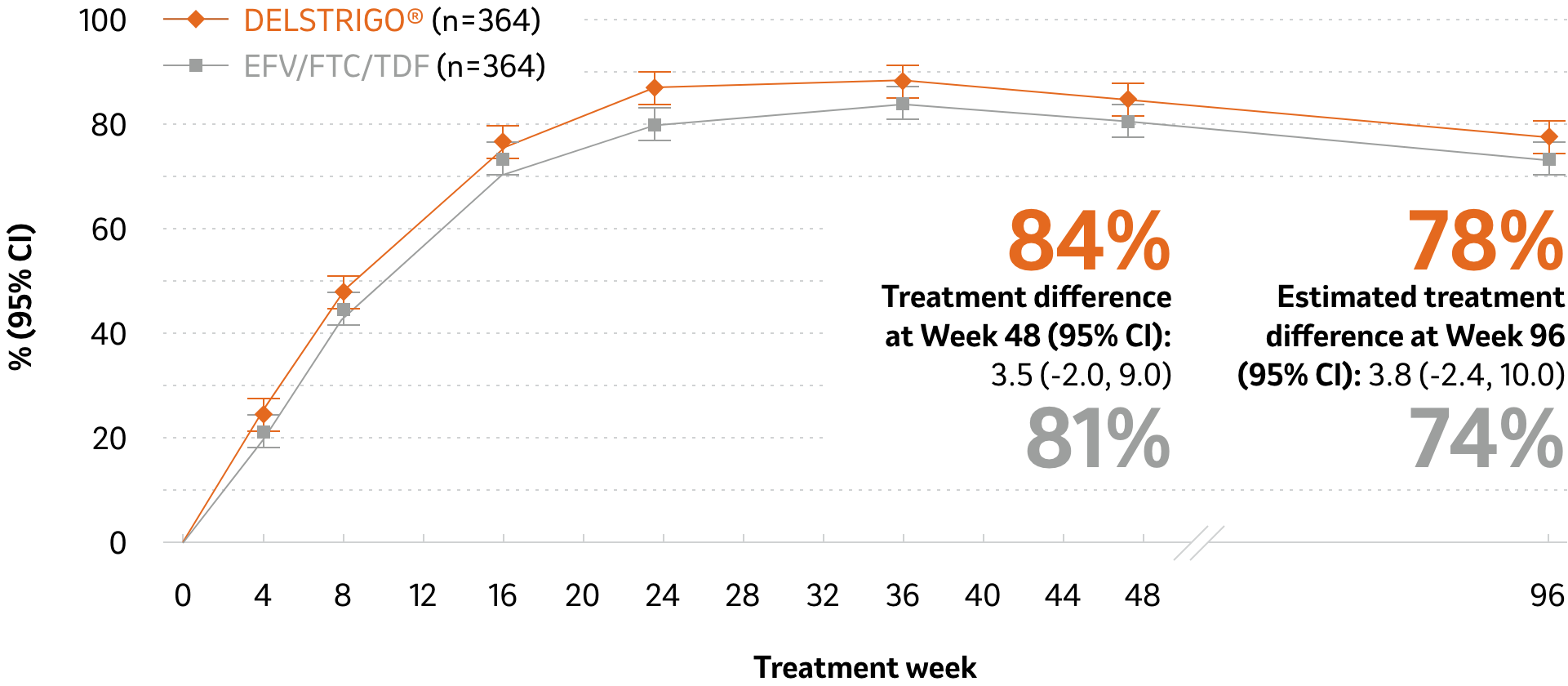
Adapted from Orkin C et al1 and DELSTRIGO®
Product Monograph2
See demographic and baseline prognostic factors
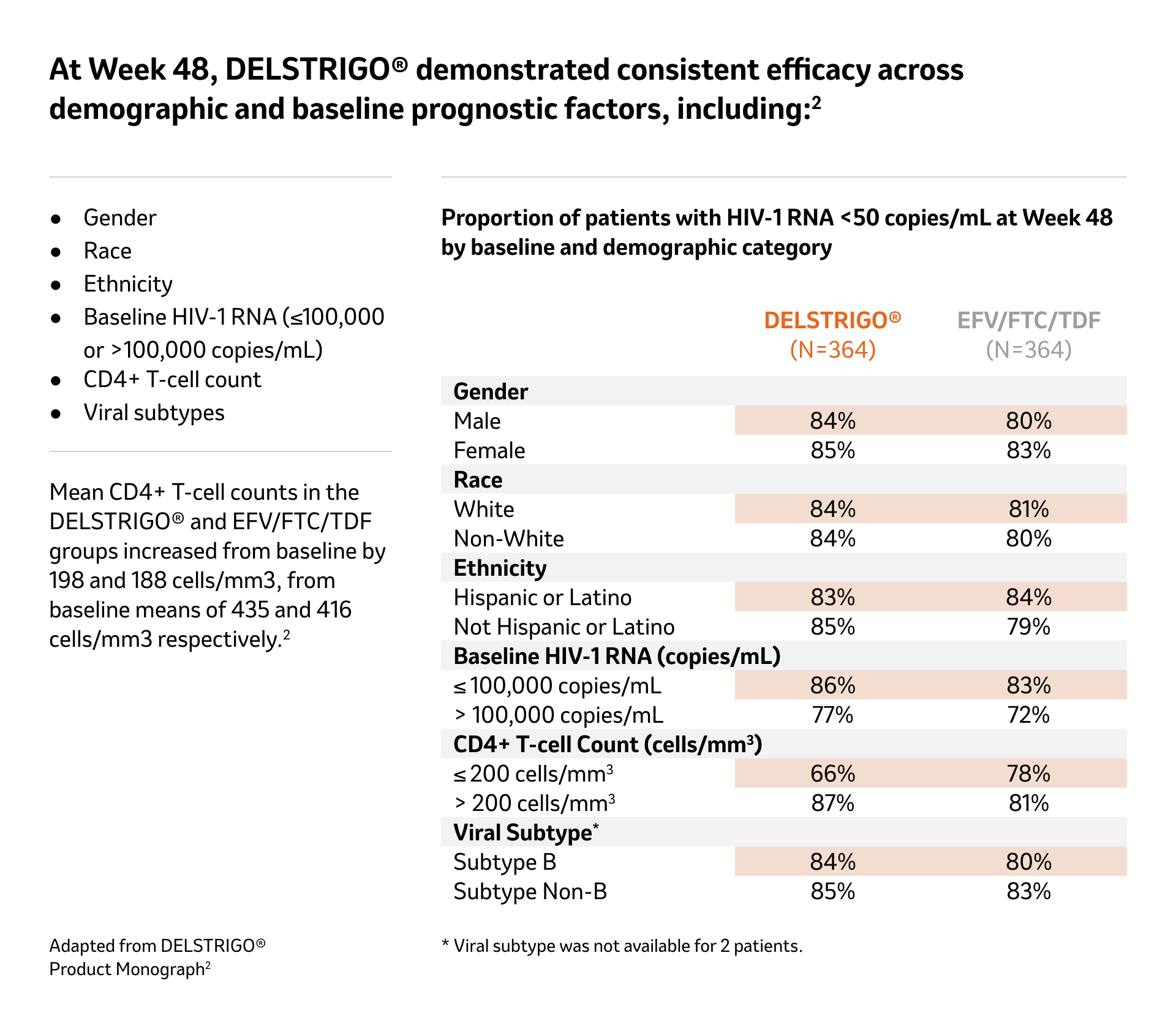
Efficacy data in patients with baseline HIV-1 of ≤100,000 or >100,000 copies/mL (secondary endpoint) at 48 weeks2
DELSTRIGO® vs. EFV/FTC/TDF
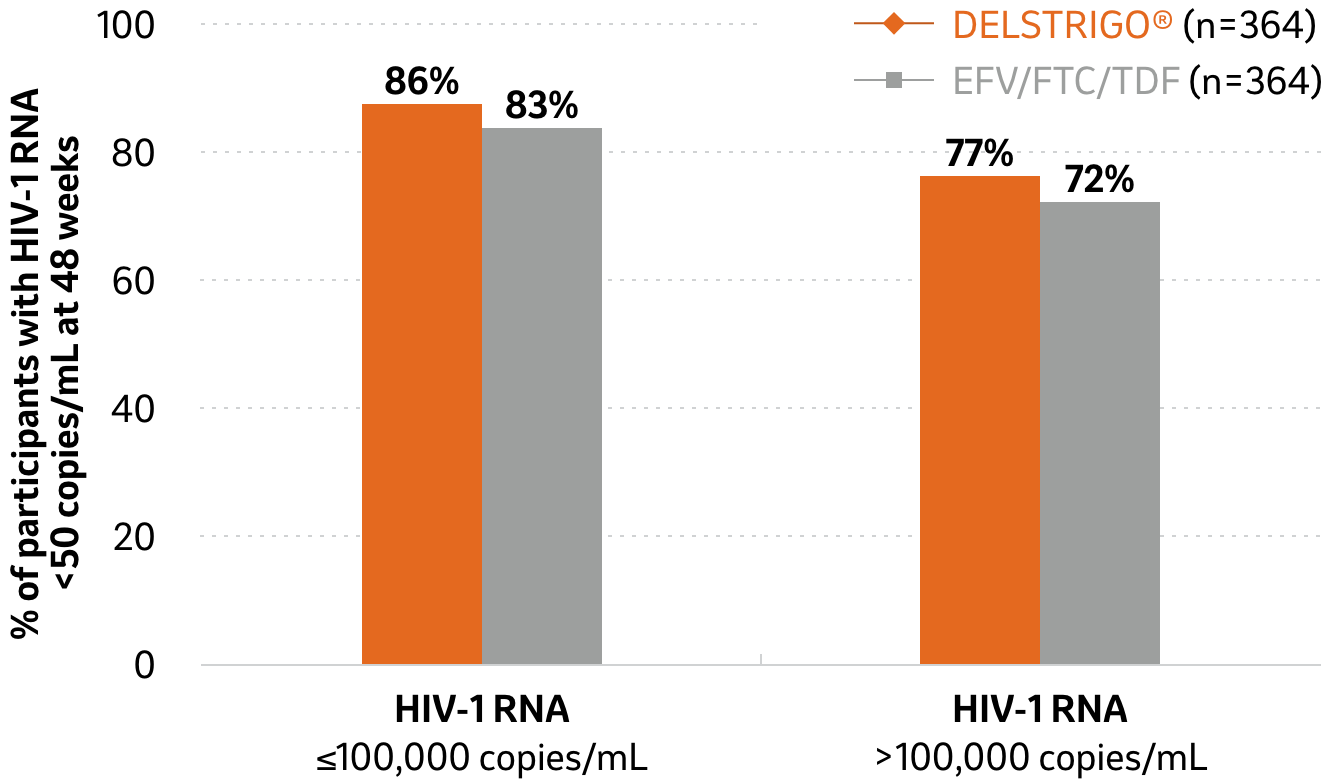
21%
of patients
at baseline had HIV-1 RNA >100,000 copies/mL1
Adapted from DELSTRIGO® Product Monograph2
* DRIVE AHEAD: Randomized, multicenter, double-blind, active-controlled 48- and 96-week trial. Adult ART-naïve patients (n=728) were randomized to DELSTRIGO® or EFV/FTC/TDF orally, once daily. DELSTRIGO® and matching placebo were taken without regard to food, at approximately the same time each day. EFV/FTC/TDF and matching placebo were taken at bedtime, on an empty stomach. [Orkin h(2)] The prespecified non-inferiority margin at week 48 was -10%.
3TC=lamivudine; TDF=tenofovir disoproxil fumarate; EFV=efavirenz; FTC=emtricitabine; FDA=Food and Drug Administration; CI=confidence interval; ART=antiretroviral therapy
References: 1. Orkin C et al. Doravirine/lamivudine/tenofovir disoproxil fumarate is non-inferior to efavirenz/emtricitabine/tenofovir disoproxil fumarate in treatment-naïve adults with Human Immunodeficiency Virus-1 Infection: Week 48 results of the DRIVE-AHEAD trial. Clin Infect Dis 2019 68(4):535-544. doi:10.1093/cid/ciy540. 2. DELSTRIGO® Product Monograph. Merck Canada Inc. December 10, 2024.
CA-DOR-00155