DRIVE-AHEAD tolerability profile
DELSTRIGO® DRIVE-AHEAD tolerability profile
DELSTRIGO® showed a superior LDL-C and non-HDL-C profile vs. EFV/FTC/TDF1
Mean change in lipid values at Week 48
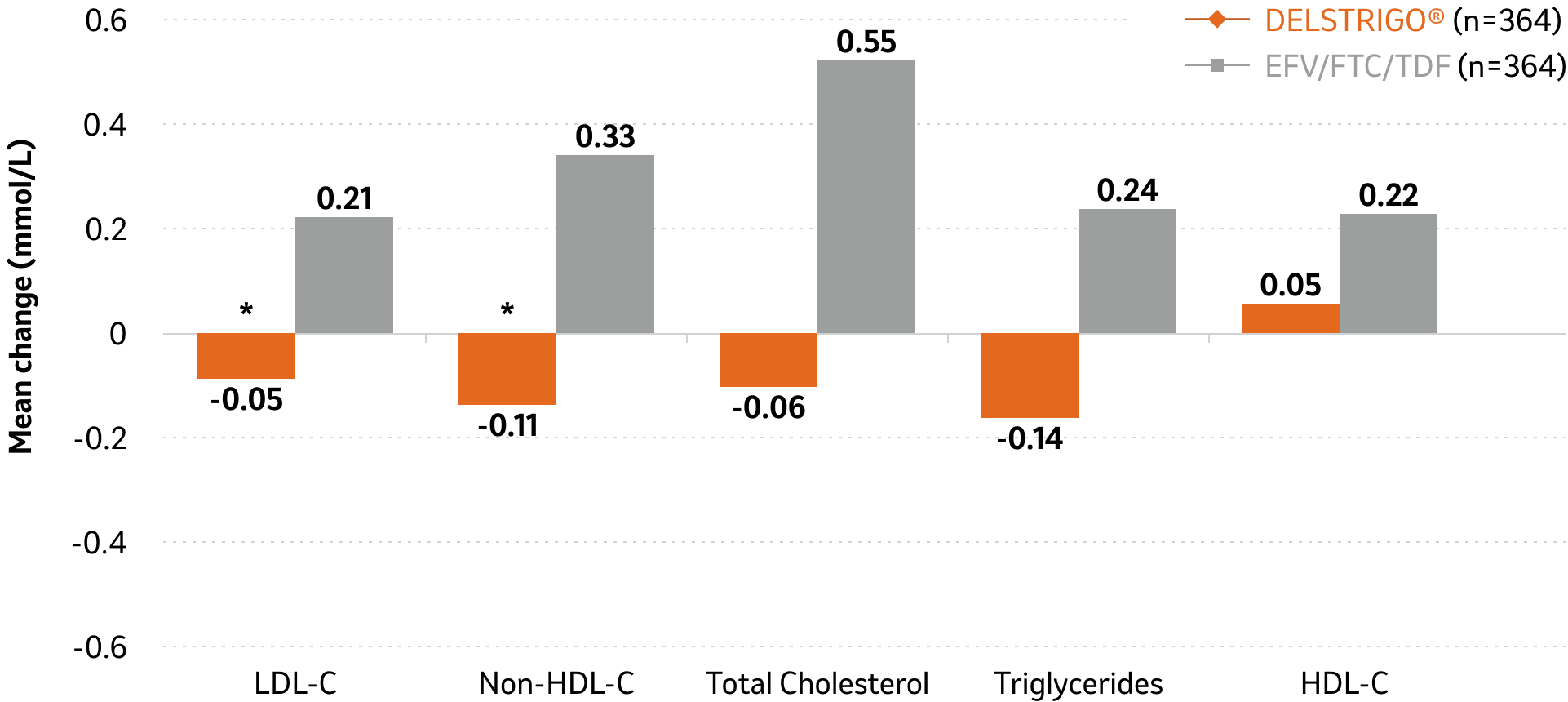
DELSTRIGO® had a neutral effect on LDL- and non-HDL-C, total cholesterol, and triglycerides, as indicated by the differences in the mean change from baseline at Week 481
Changes from baseline at Week 96 were similar to those at Week 48.1
Serum lipid levels may increase during ART. Disease control and lifestyle changes may also be contributing factors. Consideration should be given to the measurement of serum lipids. Lipid disorders should be managed as clinically appropriate.1
Patients on lipid-lowering agents at baseline were excluded from these analyses (DELSTRIGO® n=15; EFV/FTC/TDF n=10). Patients initiating a lipid-lowering agent post-baseline had their last fasted on-treatment value (prior to starting the agent) carried forward (DELSTRIGO® n=3; EFV/FTC/TDF n=8).
*P<0.0001 for pre-specified hypothesis testing for treatment differences.
Adapted from DELSTRIGO® Product Monograph1
Demonstrated rate of discontinuations due to adverse events in the 48-week results of the DRIVE-AHEAD trial in antiretroviral treatment-naïve adults with HIV-11
3.0% of patients in the DELSTRIGO™ group and 6.6% of patients in the EFV/FTC/TDF group discontinued treatment due to adverse events by Week 48.1
Rate of discontinuations through 48 weeks

Adapted from DELSTRIGO® Product Monograph1
DELSTRIGO® was shown to be generally well tolerated in the 48- and 96-week results of the DRIVE-AHEAD trial in antiretroviral treatment-naïve adults with HIV-11
Adverse reactions (all grades) reported in >2% of patients in either treatment group in ART-naïve patients at Week 48
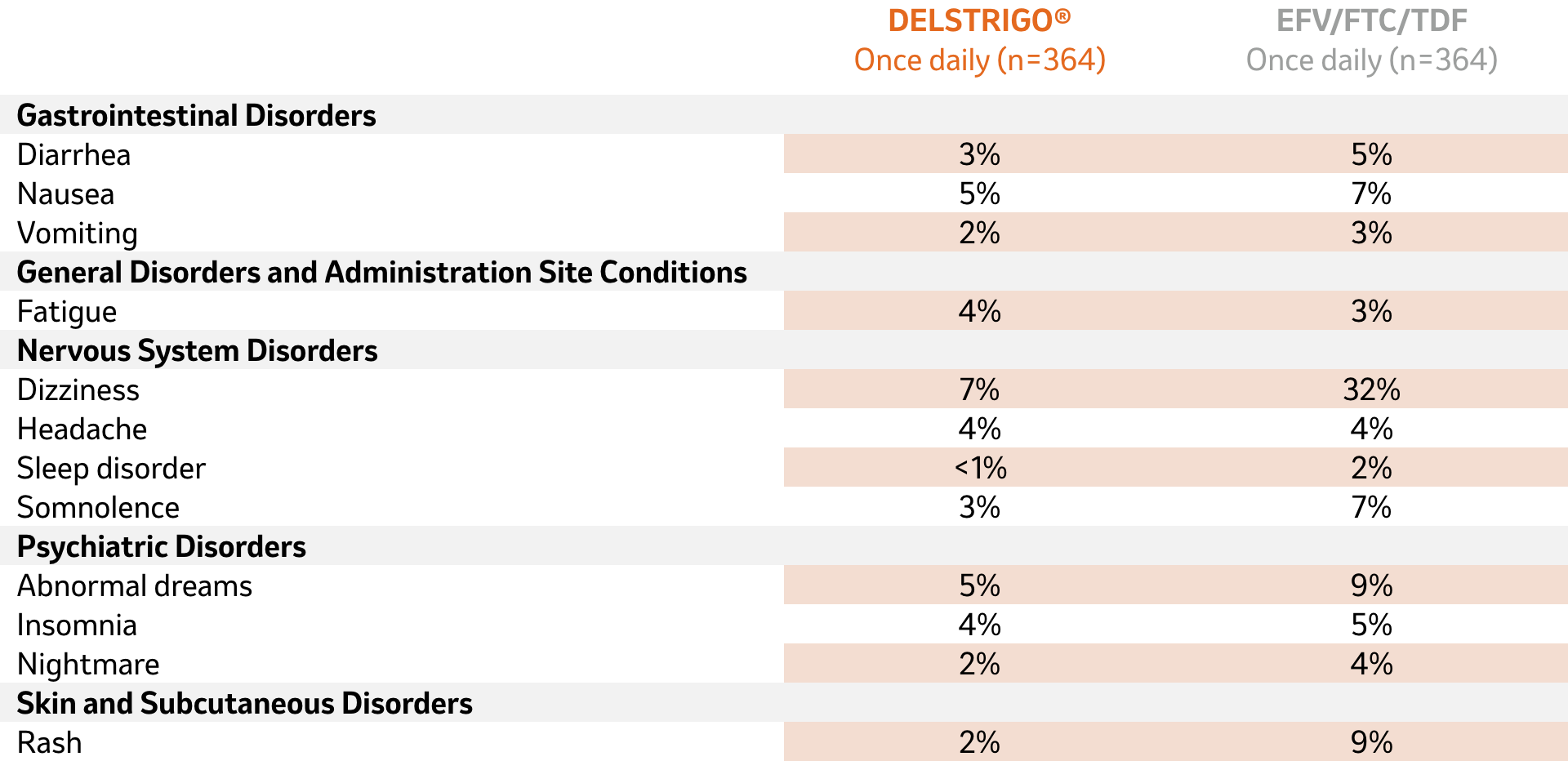
Adapted from DELSTRIGO® Product Monograph1
No adverse reactions of ≥Grade 2 (moderate or severe) occurred in ≥ 2% of subjects1
Overall, the clinical adverse experiences at Week 96 were consistent with those observed at Week 48
EFV=efavirenz; FTC=emtricitabine; TDF=tenofovir disoproxil fumarate; ART=antiretroviral therapy; LDL-C=low-density lipoprotein cholesterol; HDL-C=high-density lipoprotein cholesterol
Reference: 1. DELSTRIGO® Product Monograph. Merck Canada Inc. December 10, 2024.
CA-DOR-00155