DRIVE-SHIFT trial
DELSTRIGO® DRIVE-SHIFT trial*
DELSTRIGO® was studied in a Phase 3 non-inferiority trial in virologically-suppressed patients1,*
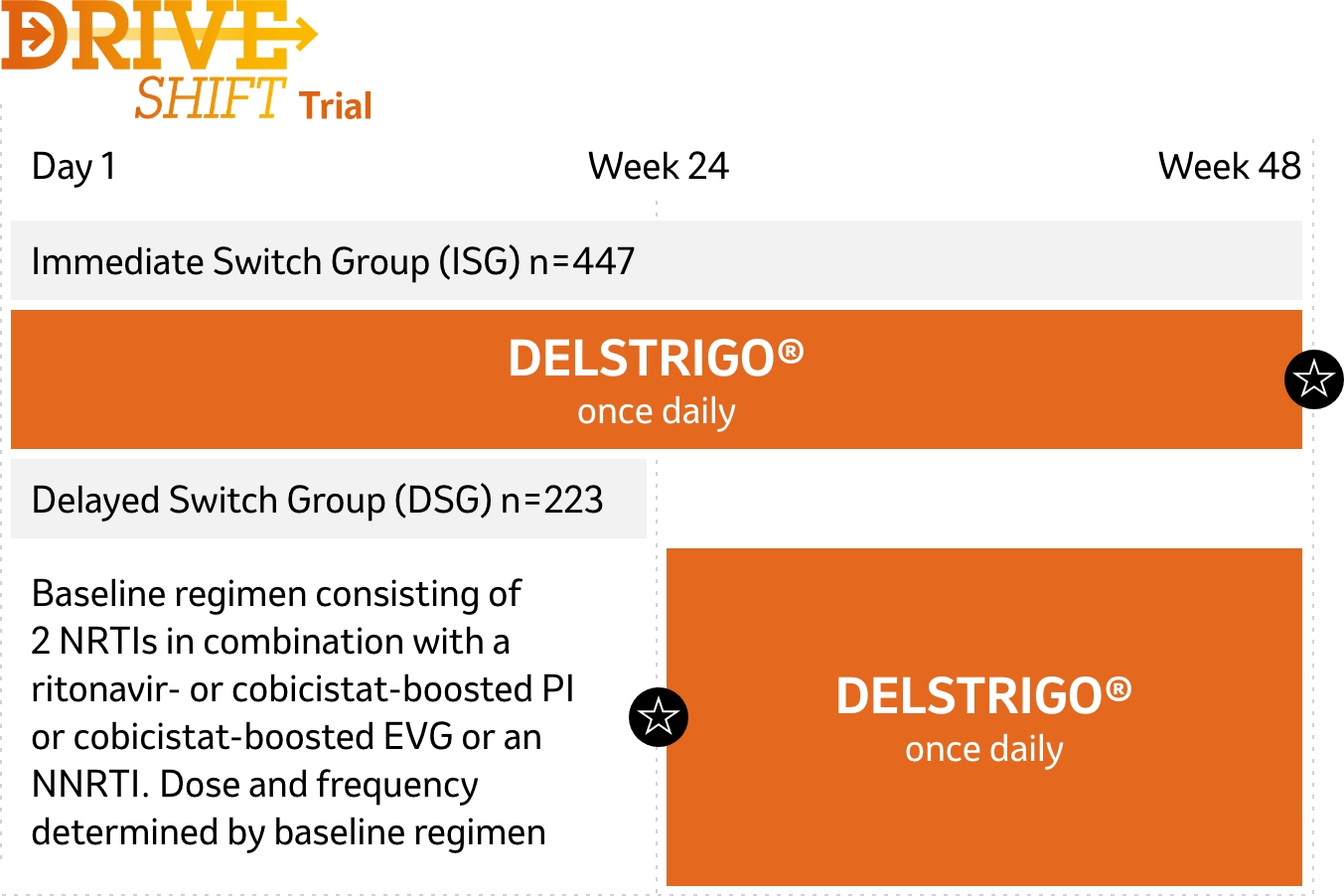
DRIVE-SHIFT was a randomized, multicenter, open-label, active-controlled, non-inferiority trial. Patients who demonstrated virologic suppression on ART for ≥6 months were randomized to switch to DELSTRIGO® immediately (immediate switch group, ISG; n=447) or to continue the baseline regimen until Week 24 and then switch to DELSTRIGO® (delayed switch group, DSG; n=223). The baseline regimen consisted of 2 NRTIs + an NNRTI (24%), a PI (70%), or cobicistat-boosted elvitegravir (an INSTI) (6%).1,2,*
Entry criteria:2
- Virologically suppressed (HIV-1 RNA <50 copies/mL) on baseline regimen for ≥6 months prior to trial entry
- No history of virologic failure
![]() Time of evaluation of primary endpoint for each treatment group
Time of evaluation of primary endpoint for each treatment group
Adapted from Johnson M et al.1 and DELSTRIGO® Product Monograph2
DELSTRIGO® ISG at Week 48 was demonstrated non-inferior to the continued baseline regimen DSG at Week 24 in maintaining viral suppression (HIV RNA <50 copies/mL)2
Proportion of patients with HIV RNA <50 copies/mL

Difference
(95% CI):
-3.8% (-7.9, 0.3)
Consistent results with the primary endpoint was seen for the comparison at Week 24 in each treatment group (secondary endpoint).2
DELSTRIGO® was non-inferior to the baseline regimen if the lower bound of the 95% CI was above -8 percentage points.
Adapted from DELSTRIGO® Product Monograph2
DELSTRIGO® as studied in the Phase 3 extension of the DRIVE-SHIFT* trial in virologically suppressed patients through Week 1443
At week 144
HIV-1 RNA <50 copies/mL:
80.1% of the ISG (n=351/438)
83.7% of the DSG (175/209)
Tolerability profile3
- The mean weight change from switch to Week 144 was +1.4 kg for ISG and +1.2 kg for DSG
Safety profile3,4
- The most common adverse events were nasopharyngitis (16.2%), headache (12.3%), and diarrhea (9.1%). Overall, 4.1% discontinued because of adverse events
The duration of this study is longer than that of the data in the Product Monograph.
* DRIVE-SHIFT: Randomized, international, multicenter, open-label, active-controlled, non-inferiority trial. Patients who demonstrated virologic suppression on ART for ≥6 months were randomized to switch to DELSTRIGO® immediately (immediate switch group, ISG; n=447) or to continue the baseline regimen until Week 24 and then switch to DELSTRIGO® (delayed switch group, DSG; n=223). The primary endpoint (snapshot approach) was the proportion of participants with HIV-1 RNA <50 copies/mL, with the primary comparison between the ISG at Week 48 vs. the DSG at Week 24. DELSTRIGO® was non-inferior to the baseline regimen if the lower bound of the 95% CI was above -8 percentage points. Baseline ART was a ritonavir- or cobicistat-boosted PI (atazanavir, darunavir or lopinavir), cobicistat-boosted elvitegravir, or an NNRTI (efavirenz, nevirapine, or rilpivirine), each in combination with two NRTIs. Participants who completed the Week 48 visit were eligible to enter study extension-1 and continue receiving open-label DOR/3TC/TDF for an additional 96 weeks (up to Week 144). Participants with protocol-defined virologic failure were discontinued from the trial, regardless of adherence to study therapy.
ART=antiretroviral therapy; DOR/3TC/TDF=doravirine/lamivudine/tenofovir disoproxil fumarate; DSG=Delayed Switch Group; ISG=Immediate Switch Group; NRTI=nucleoside reverse transcriptase inhibitor; NNRTI=non-nucleoside reverse transcriptase inhibitor; PI=protease inhibitor; INSTI=integrase strand transfer inhibitor; EVG=elvitegravir; CI=confidence interval
References: 1. Johnson M et al. Switching to doravirine/lamivudine/tenofovir disoproxil fumarate (DOR/3TC/TDF) maintains HIV-1 virologic suppression through 48 weeks: results of the DRIVE-SHIFT trial. J Acquir Immune Defic Syndr 2019;81:463-72. 2. DELSTRIGO® Product Monograph. Merck Canada Inc. December 10, 2024. 3. Kumar P et al. Switching to DOR/3TC/TDF Maintains HIV-1 Virologic Suppression Through Week 144 in the DRIVE-SHIFT Trial. J Acquir Immune Defic Syndr 2021;87(2):801-805. 4. Medical Regulatory Letter. Merck Canada. September 21, 2023.
CA-DOR-00155