Drug-drug interactions
Drug-drug interaction profile
PIFELTRO® drug-drug interactions*
Doravirine is primarily metabolized by CYP3A, and may be affected by its inducers or inhibitors. At 100 mg once daily, doravirine is unlikely to have a clinically relevant effect on drugs metabolized by CYP enzymes.1
PIFELTRO® is contraindicated with drugs that are strong cytochrome P450 (CYP)3A enzyme inducers.1
No dose adjustments required for either drug when doravirine is co-administered with these drugs:1,†

No clinically relevant interactions are expected when doravirine is co-administered with the following drugs:1
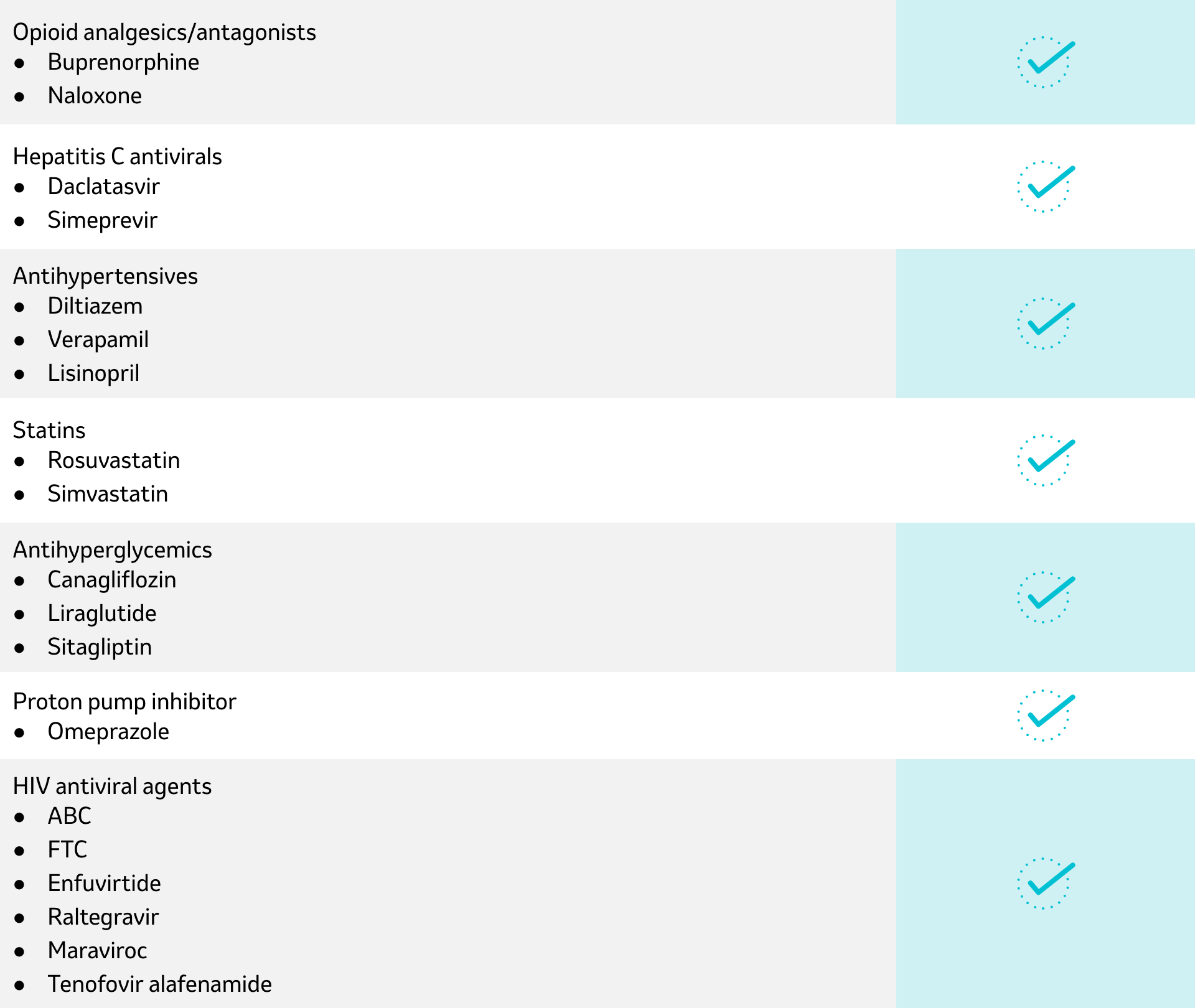
Established and other potentially significant drug interactions (not inclusive): alterations in dose or regimen may be recommended based on drug interaction studies or predicted interaction
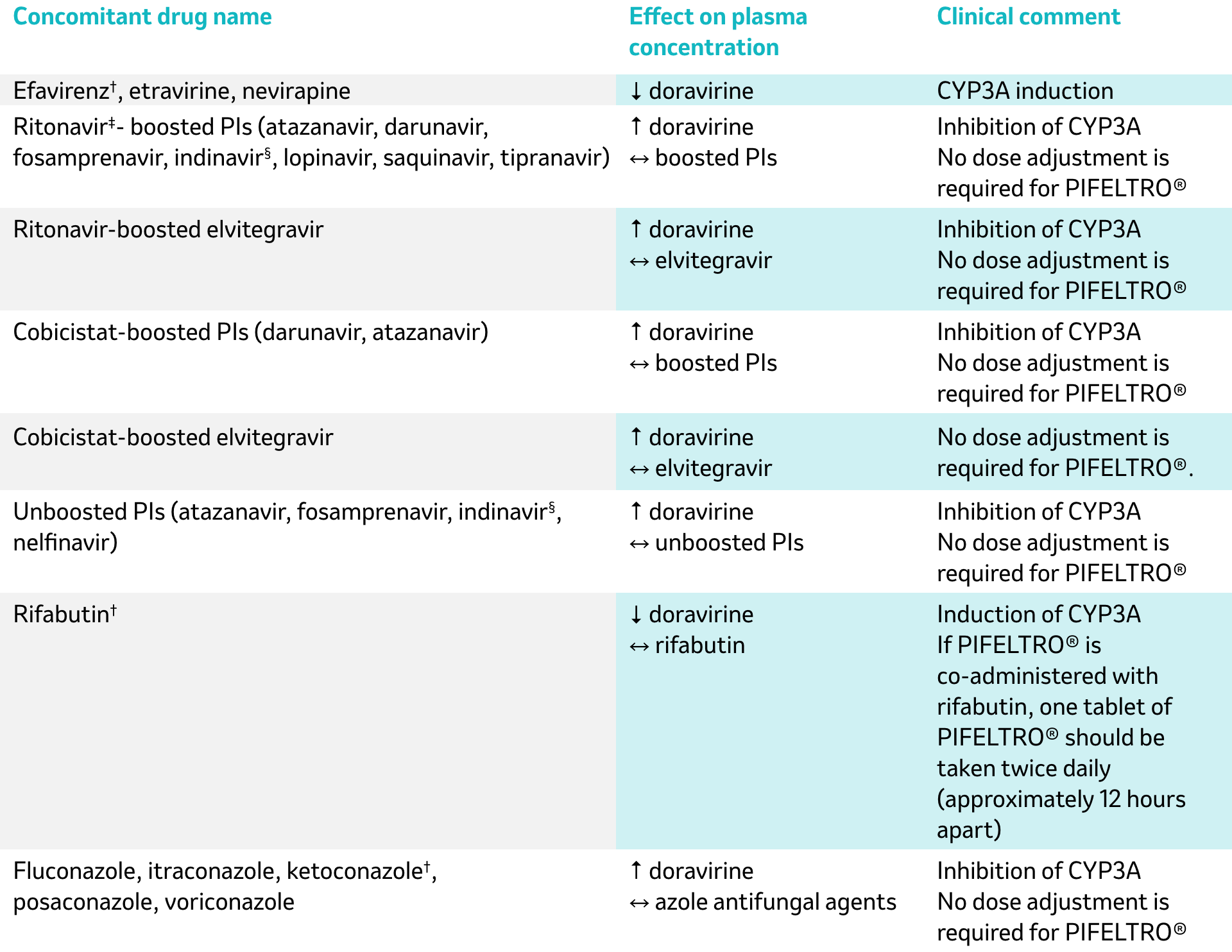
All drug-drug interactions shown, other than those footnoted, are anticipated based on the known metabolic and elimination pathways.
Adapted from PIFELTRO® Product Monograph1
Changes in Pk values of doravirine in the presence of co-administered drug

Adapted from PIFELTRO® Product Monograph1
Changes in Pk values of co-administered drugs in the presence of doravirine
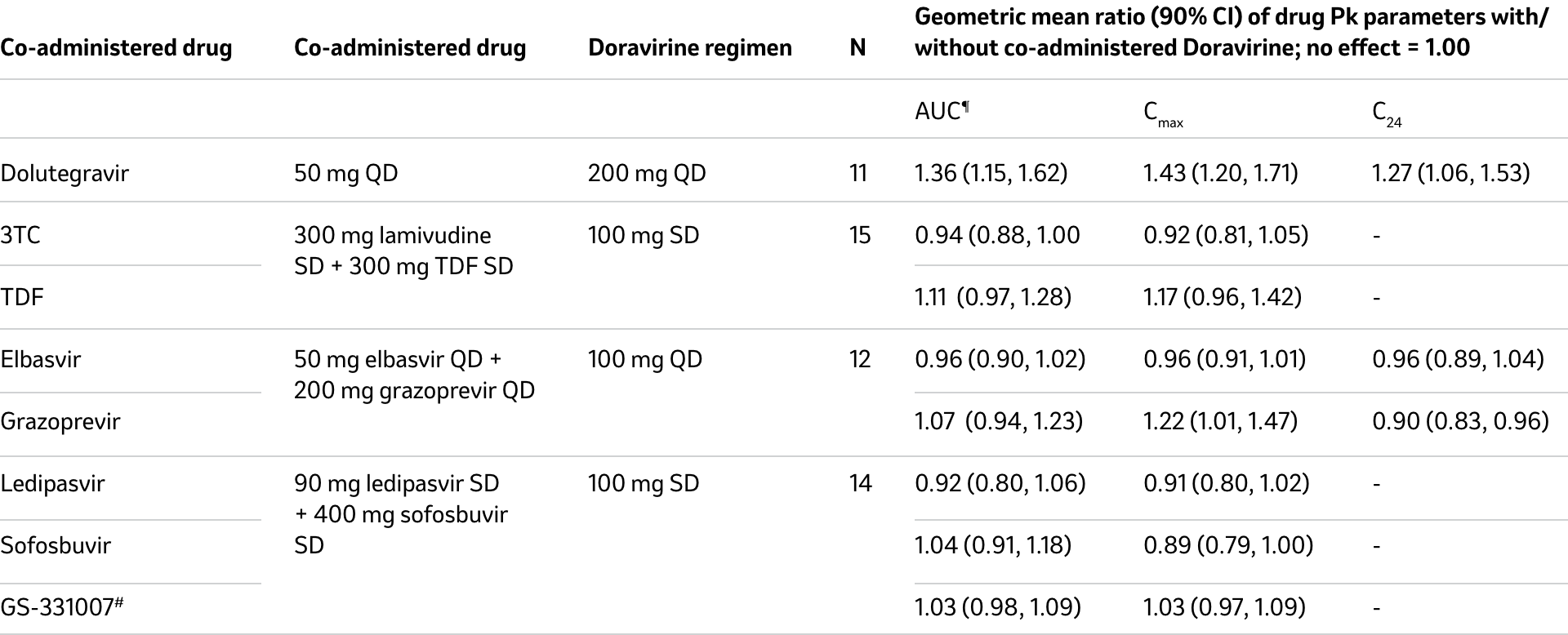
Adapted from PIFELTRO® Product Monograph1
* Please consult the Product Monograph for a complete list.
† Evaluated in a clinical trial.
‡ Evaluated with ritonavir only.
§ Not marketed in Canada.
¶ AUC0-∞ for single-dose, AUC0-24 for once daily.
# The predominant circulating nucleoside metabolite of sofosbuvir.
3TC= lamivudine; TDF=tenofovir disoproxil fumarate; ABC=abacavir; FTC= emtricitabine; PI=protease inhibitor; Pk=pharmacokinetic; CI=confidence interval; SD=single dose; QD=once daily; BID=twice daily
Reference: 1. PIFELTRO® Product Monograph. Merck Canada Inc. December 10, 2024.
CA-DOR-00155